Abstract
Introduction: While maintenance rituximab monotherapy has been shown to improve progression free survival in select lymphoma settings, current guidelines do not support extending maintenance beyond 2 years. Limited studies outside the setting of hematologic malignancies suggest financial incentives many influence utilization of infusional therapies in the United States. We therefore set out to determine whether patients treated by community providers, who may have direct financial incentive to provide additional rituximab, were more likely to receive non-guideline based extended maintenance compared to those patients treated by hospital-employed providers.
Methods: Using the SEER-Medicare database, we identified older adults diagnosed from 2004 through 2011 with B cell non-Hodgkin lymphoma who had at least one claim for rituximab through 2013. Patients were included if they had at least 7+ months with rituximab claims without a 200-day gap between rituximab claims. The first rituximab monotherapy claim at 7+ months following rituximab initiation was defined as the index date for maintenance rituximab. Both the number of rituximab monotherapy claims and the duration of rituximab maintenance were calculated for each beneficiary until receipt of chemotherapy, 200-day gap in rituximab claims, or death. The site of rituximab administration was classified as community or hospital-outpatient as indicated on Medicare claims. We used a logistic regression model to assess the independent association between setting of care and prolonged rituximab use, adjusting for age, sex, histology, year of diagnosis, sociodemographics, Medicaid status, comorbidity score, and performance status.
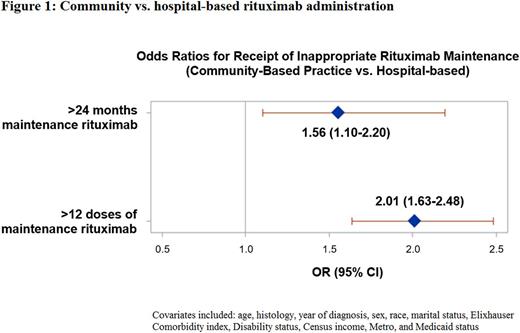
Results: We identified a total of 2,620 patients with >2 years of available follow up after their initiation of rituximab maintenance. The median age was 75 years (IQR 70-80) and the majority were white (94.5%) and female (53.6%). Three-quarters of patients (75.1%) received their maintenance rituximab in the community setting. Follicular lymphoma was the most common histology (39.7%) followed by diffuse large B cell lymphoma (18.8%). The median number of rituximab maintenance doses received was 9 (range 1-103 doses). The median duration of maintenance was 14 months (range 0-92), with 261 (10.0%) patients receiving uninterrupted rituximab maintenance for greater than 2 years. Patients in the community setting were more likely to receive >2 years of rituximab maintenance (11.0% community vs. 6.9% hospital, p = .003). In multivariable logistic regression (Figure 1), treatment in the community setting was significantly associated with receipt of >2 years of maintenance (adjusted OR = 1.56, 95% CI 1.10-2.20; p = .012) and >12 doses of rituximab monotherapy (adjusted OR = 2.01, 95% CI 1.63-2.48; p <.001). Findings were similar on sensitivity analyses using the outcomes of >30 months and >18 doses of rituximab monotherapy.
Conclusion: Receipt of lymphoma care in the community setting was associated with guideline-discordant use of rituximab. Since financial incentives in the community setting are more likely to be strongly tied to administration of anti-cancer therapies, our findings suggest financial incentives may contribute to overutilization during rituximab maintenance.
Huntington: Pharmacyclics: Honoraria; Janssen: Consultancy; Celgene: Consultancy, Other: Travel. Gross: Pfizer: Research Funding; Johnson and Johnson: Research Funding; 21st Century Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal